The BGMA is calling for a coordinated industrial strategy to safeguard the supply of generic and biosimilar medicines in the UK
The Covid-19 pandemic has challenged all areas of society. The impact has been particularly acute in the healthcare system, with unprecedented demand for medicines globally.
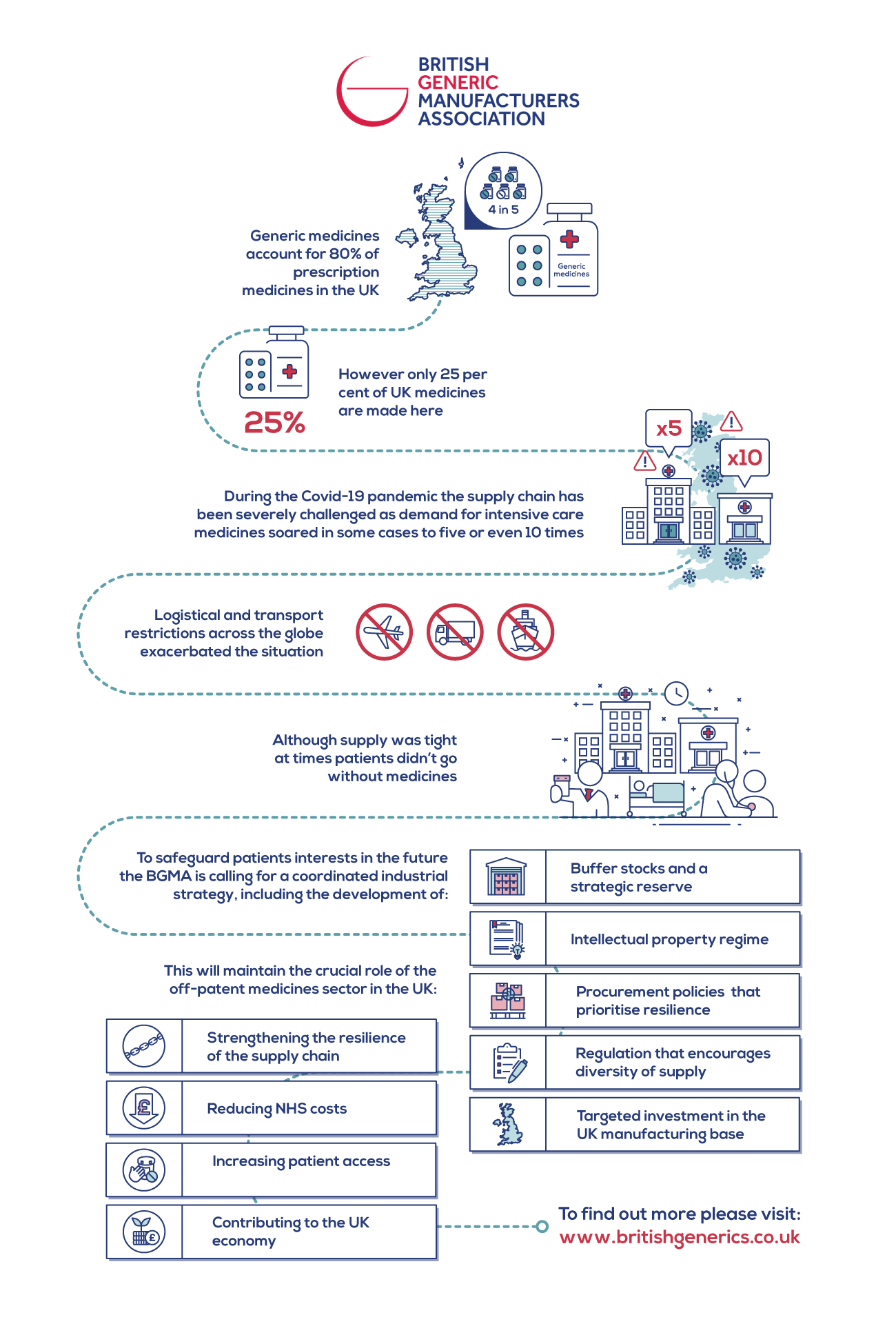
Generic medicines, which make up four in every five prescription medicines in the UK, were central to the treatment of hospitalised patients placed on mechanical ventilation, impacted during the peak of the first wave of the pandemic.
The Generics medicines industry exists to promote innovation, reduce costs, increase supply, and increase patient access, ensuring that products get to more patients in the right place at the right time. However, this objective was severely challenged during the pandemic as demand for intensive care medicines soared in some cases to five or even 10 times, compared to usual levels. This, coupled with logistical and transport restrictions across the globe meant manufacturers - along with the NHS, clinicians and the Government - had to work extremely hard not only to fulfil orders but ensure they made it to the UK. Although supply was tight at times, patients did not go without their medicines.
In the wake of the Covid crisis many lessons have been learned, however the threat remains and preparations must continue to be made to mitigate and prepare for future similar events.
That’s why the BGMA is putting forward recommendations for a coordinated industrial strategy to safeguard the supply of generic and biosimilar medicines in the UK and safeguard patients interests in the future. These recommendations include:
- Production of buffer stocks and a strategic reserve for the most important medicines
- An intellectual property regime that attracts generic and biosimilar manufacturers to the UK
- Procurement policies that prioritise resilience as well as cost
- Regulation that encourages diversity of supply
- Targeted investment in the UK manufacturing base.
These recommendations form a strategic approach to maintaining the crucial role of the off-patent medicines sector in the UK, strengthening the resilience of their supply chain, and ensuring their continued contribution to reducing NHS costs, increasing patient access, and contributing to the UK economy.
We have distilled our thinking into two companion reports. To find out more about how our sector supported the NHS during the first peak of the pandemic and the lessons that have been learned, then Download our ‘Strengthening the Resilience of the Global Supply Chain’ report. To find out why our sector needs a bespoke industrial strategy Download our ‘Generic Industry Supply Chain Resilience Post-Covid-19’ report, where you can read our policy perspectives in more detail.
Strengthening the resilience of the global supply chain

Generic industry supply chain resilience post-COVID-19