Back to News index
Medicines UK supply issues dashboard
Looking at August 2021, Medicines UK provides a monthly snapshot of the numbers of supply issues affecting generic[1] medicines in England, based on UK Government and NHS data.
Supply issues as a proportion of presentations listed in the Drug Tariff[2]
0.03%
Proportion of Serious Shortage Protocols[3] in force for generic medicines as a proportion of the number of presentations listed in the Drug Tariff
0.14%
Proportion of Medicines Supply Notifications[4] issued in the last month[5] for generic medicines as a proportion of the number of presentations listed in the Drug Tariff
0.03%
Proportion of Supply Disruption Alerts[6] issued in the last month[7] for generic medicines as a proportion of the number of presentations listed in the Drug Tariff
1.2%
Proportion of generic medicines listed in the Department of Health and Social Care (DHSC) August 2021 supply issues update[8] as a proportion of the number of presentations listed in the Drug Tariff
1.0%
Proportion of generic medicines listed in the DHSC August 2021 supply issues update where no equivalent licensed alternative[9] is available as a proportion of the number of presentations listed in the Drug Tariff
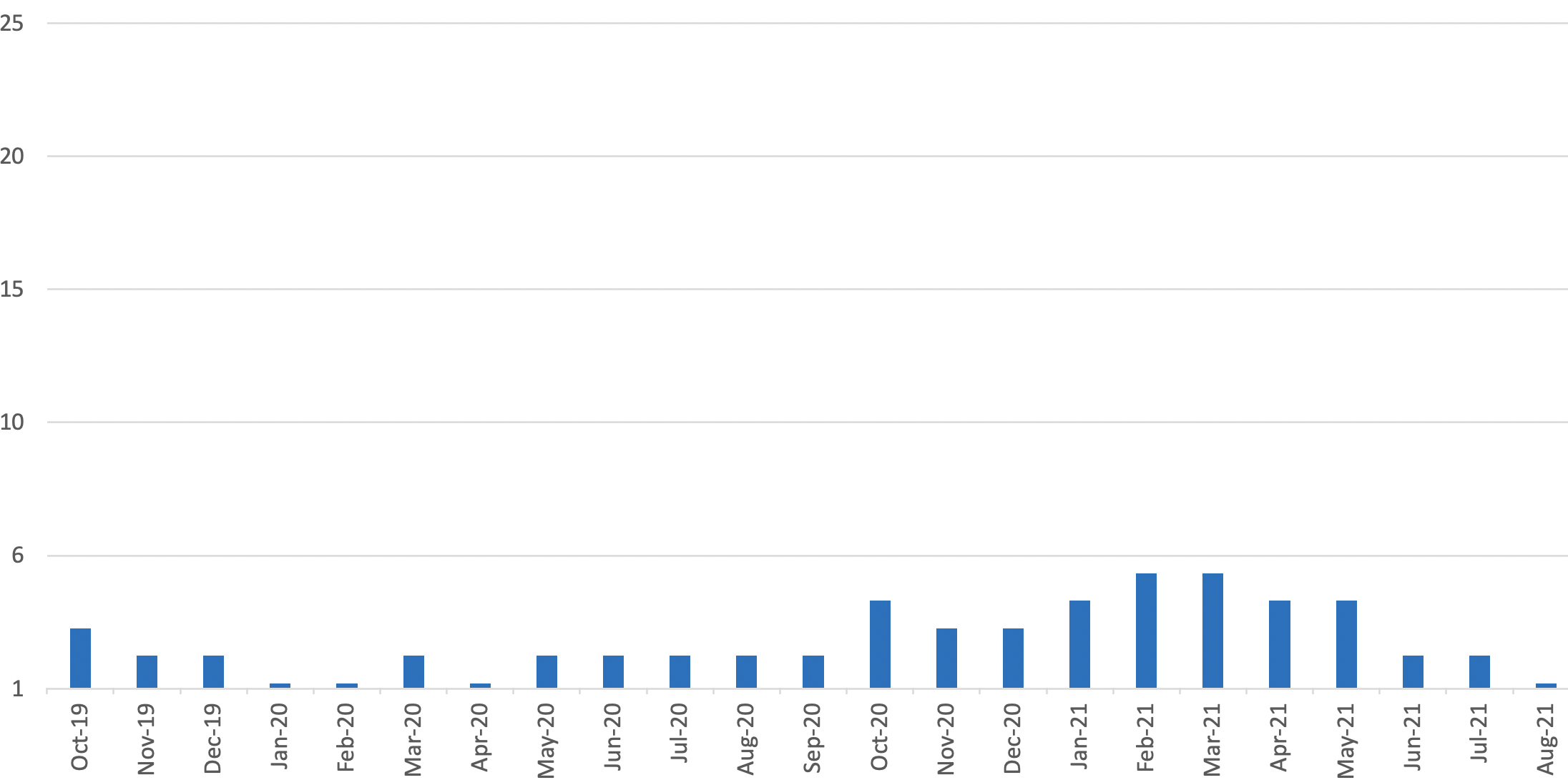
Serious Shortage Protocols in place covering any days in the month
- Number of generic presentations for which a Serious Shortage Protocol is currently in place = 1

- Number of molecules for which a Serious Shortage Protocol has been in place at any time since inception in October 2019 = 8
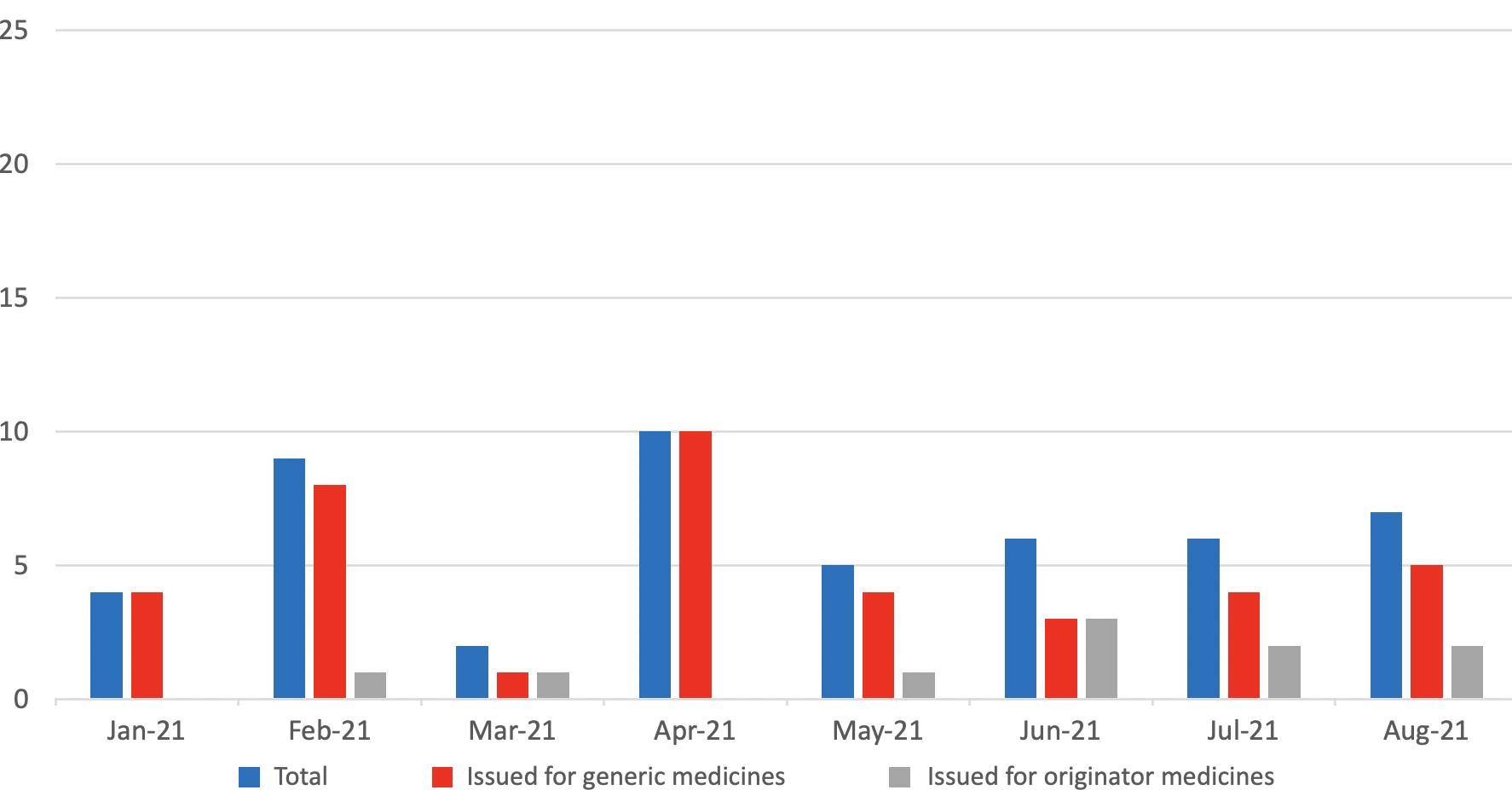
Medicine Supply Notifications issued per month

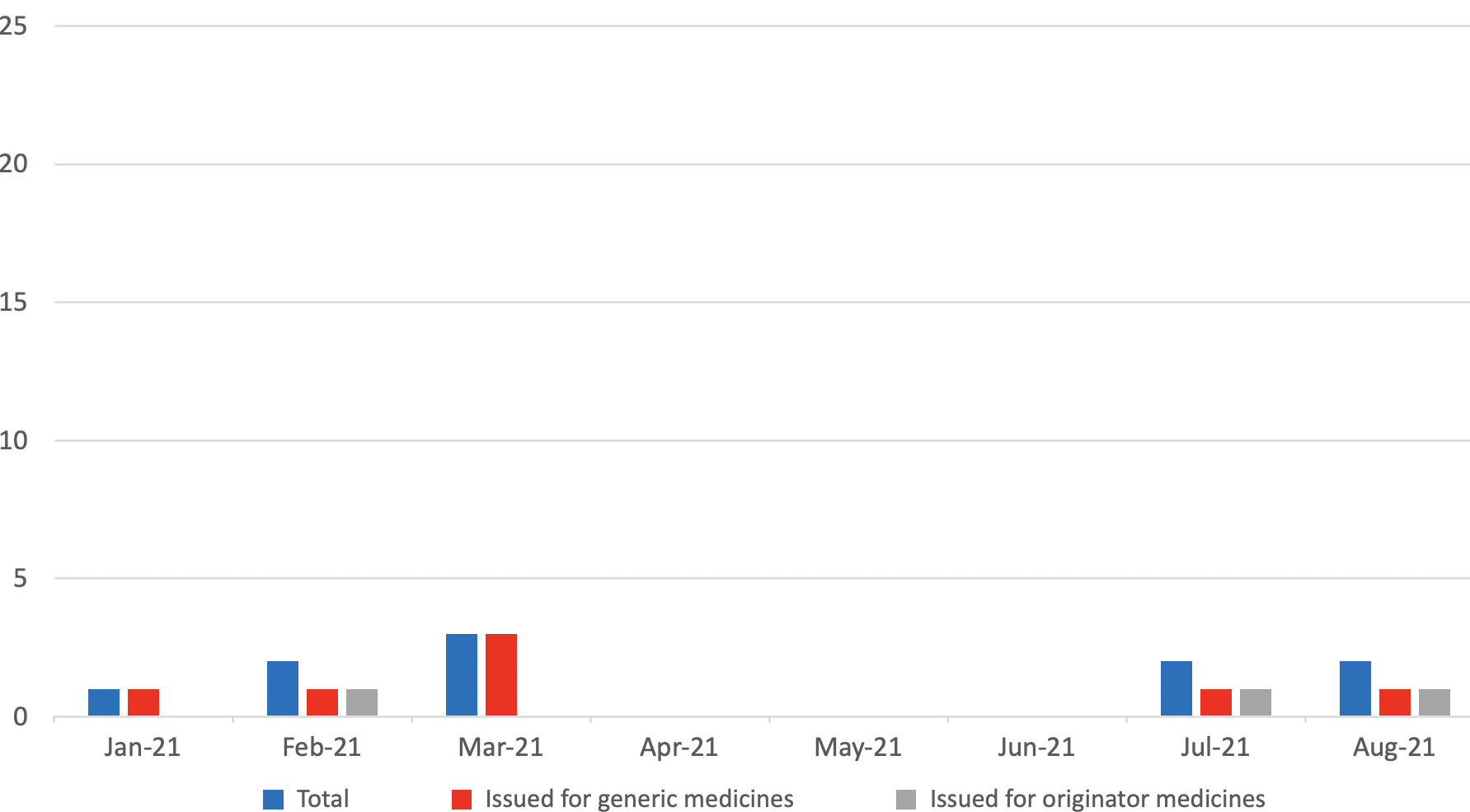
Supply Disruption Alerts issued per month

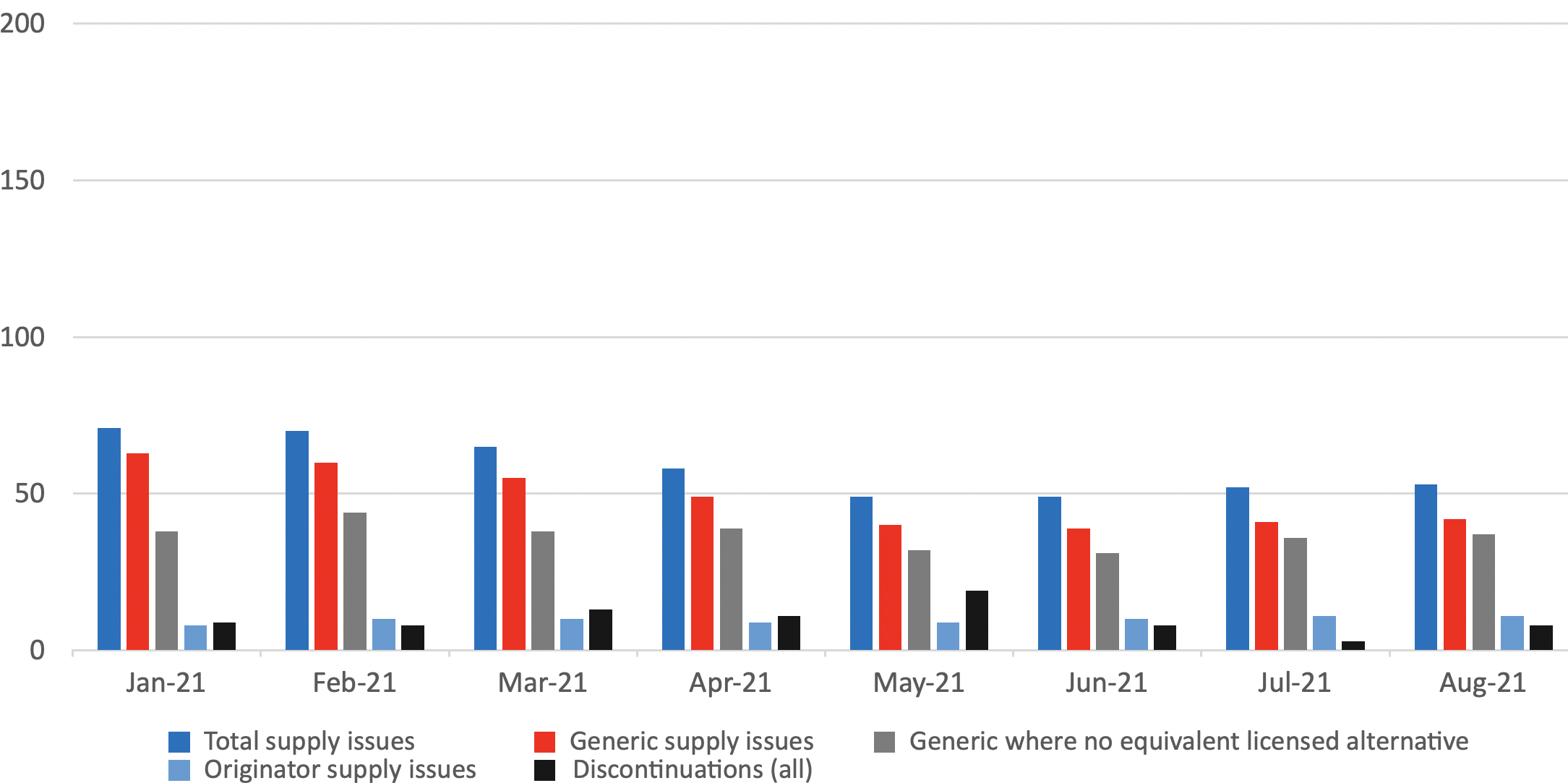
Number of supply issues and discontinuations identified in the DHSC monthly supply issues update

Presentations in the Drug Tariff
- Number of medicine presentations in August 2021 Drug Tariff = 3,918
Notes:
- 1 In this analysis, we define a generic medicine as a medicine that is not patent protected and is not solely supplied by the original inventor of the medicine. As such, off-patent originator medicines may be included as generic medicines, since they operate in genercised markets.
- 2 Listed under Part VIIIA – the Basic Prices of Drugs. Source: https://www.nhsbsa.nhs.uk/sites/default/files/2020-12/Drug%20Tariff%20January%202021.pdf. In August 2021, there were 3,918 different presentations listed in the Drug Tariff. From a sample undertaken in April 2021, we found that 10% of presentations had the same molecule, strength and form as another presentation. We have therefore cut the Drug Tariff measure by 10% to 3,526 to ensure that we are accurately measuring against the supply issues identified in the DHSC supply issues update sheet (explained below), which is compiled on the same molecule, strength and form basis.
- 3 According to the NHS Business Services Authority: "An SSP enables community pharmacists, in the event of a serious shortage of any prescribed item that affects or may affect the whole or any part of the UK, to supply in accordance with the Protocol rather than supply in accordance with a prescription, without going back to the prescriber". Source: https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/serious-shortage-protocols-ssps
- 4 Based on DHSC definitions, Medicines Supply Notifications may be issued where there are potential safety implications which may require clinical advice to the NHS and pharmacists. This may arise where: no or limited clinical alternatives exist; where the product is designated by the MHRA for the patient to be maintained on a manufacturer's brand and switching is viewed as difficult; where the process of switching a patient over to a therapeutic alternative requires monitoring; or the patient group affected is likely to be considered vulnerable.
- 5 New or revised existing MSNs published since the previous month's DHSC supply issues update are included.
- 6 Based on NHS England and DHSC definitions, Supply Disruption Alerts may be issued where a supply issue has the potential to have a more serious patient safety or system-wide impact and therefore require prompt and proactive actions for clinicians to undertake locally. SDAs may be issued for similar reasons to MSNs. In addition, SDAs may also be used where a supply gap remains following the exhaustion of supply or it is likely to have a life-threatening impact on patients and potentially requires other agencies to be aware outside the NHS and other health bodes. https://www.england.nhs.uk/wp-content/uploads/2019/11/a-guide-to-managing-medicines-supply-and-shortages-2.pdf
- 7 New or revised existing SDAs published since the previous month's DHSC supply issues update are included.
- 8 Every month, DHSC, with the help of the Commercial Medicines Unit covering secondary care, produces a consolidated list of medicines with new, ongoing or resolved supply issues that are being actively managed to prevent or mitigate patient impact. The list is circulated to pharmacists and relevant NHS bodies that prescribe or oversee the prescribing of medicines. The sheet notes the level of availability of each medicine, providing advice to prescribers where needed. This also includes notice of medicines where the supplier has confirmed that it is discontinuing supply. Regional supply issues may not be captured, such as where other suppliers can fill demand.
- 9 We have measured an equivalent licensed alternative as one where a pharmacist can make a switch to another licensed presentation without reverting to the prescriber to change the prescription. This might be where another manufacturer's product or a different pack size is available, or a parallel import is available. Even where we have listed that there is no equivalent licensed alternative, a different form or strength of the medicine may be available which the patient can be safely prescribed.
